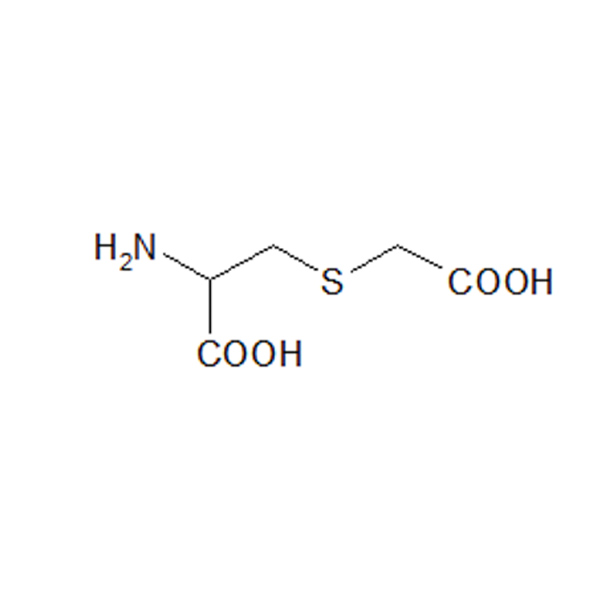
CARBOCYSTEINE
Therapeutic category: Mucolytic
Reference: 638-23-3
Standard package: Fiber drum, 50 Kg
Regulatory data: CEP, EDMF JDMF
Pharma-copoeias: Ph. Eur. 11 JP 16
Production site: CATALANA & BCN
IUPAC name: (2R)-2-Amino-3-[(carboxymethyl)sulfanyl]propanoic acid
Pharmaceutical Dose Forms:
capsule/tablet
Additional information
IUPAC name
(2R)-2-Amino-3-[(carboxymethyl)sulfanyl]propanoic acid
Pharmaceutical Dose Forms
capsule/tablet